Introduction:
Infant acute lymphoblastic leukemia (ALL), is a particularly aggressive subtype of leukemia with an early onset and unfavorable clinical outcome. Most (~70%) cases of infant ALL involve chromosomal rearrangement of KMT2A (KMT2A-r) on chromosome 11q23, the strongest independent predictor of a poor prognosis. To date, genomics studies have consistently demonstrated KMT2A-r infant ALL to have a strikingly silent landscape of DNA mutations, aside from the KMT2A-r itself. Germline mutations in cancer predisposition genes are found in 8.6% of pediatric malignancies and 4.4% of pediatric leukemias, compared to 1.1% in persons in the 1000 Genomes Project (Zhang J et al., N Engl J Med 2015). We hypothesized that germline variants may contribute to the development of KMT2A-r ALL in infants. We examined the germline variants in remission blood samples from a large cohort of infants with KMT2A-r ALL who were enrolled in Children's Oncology Group (COG) trial AALL15P1.
Methods:
We performed whole genome sequencing (WGS) and whole exome sequencing (WES) on DNA isolated from peripheral blood from 36 KMT2A-r cases at time of remission. Sequencing was performed using an Illumina Hiseq 4000 or 2500 to a minimum depth of 90Gb (WGS) and 15Gb (WES). Alignment and variant calling were performed using the Dragon Bio-IT platform (v 3.2.8, Illumina). Blueprint Genetics clinical panels and the medical literature (Xa M et al., Nature 2018) were used to comprise a list of 346 genes associated with cancer predisposition and bone marrow failure syndromes. From this gene pool, variants were selected for analysis based on a variant allele frequency of ~50% and minor allele frequency <0.1% in control population databases (gnomAD). Variants were analyzed for pathogenicity per the 2015 ACMG/AMP interpretation guidelines for sequence variants.
Results:
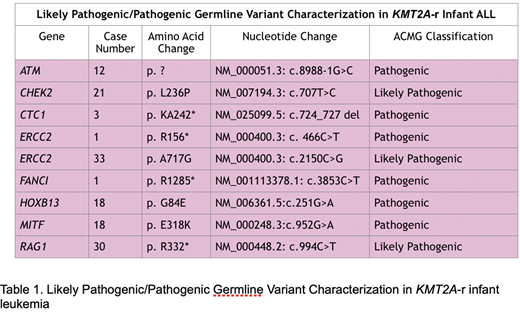
Of 351 variants initially identified, we found 3 likely pathogenic (LP) and 6 pathogenic(P) non-synonymous germline variants (for a total of 9 LP/P variants) and 144 variants of unknown significance (VUS). In total, 19.4% (n=7) of patient samples displayed at least one LP/P variant. Two patient samples contained 2 variants each. Variants classified as VUS, LP, or P were further characterized by possible causative pathway: 37.9% (n=58) of variants were in genes associated with bone marrow failure (BMF), 17.6% (n=27) in driver genes, 13.1% (n=20) in genes associated with inherited leukemias, 11.1% (n=17) in tumor suppressor genes, 7.8% (n=12) in tyrosine kinase genes, and 29.4% (n=45) in other predisposition genes . Many variants were present in more than one pathway and are represented as such. Table 1 demonstrates the genetic characteristics of the 9 P/LP variants found in our cohort. ERCC2 was the only gene with multiple LP/P variants across samples, accounting for 2 (1 LP, 1 P) of the 9 deleterious variants identified (22%).
Conclusion:
We identified germline variants in cancer predisposition genes in 19.4% of this cohort of infant ALL patients, a higher mutation rate than has previously been reported. Among pathways evaluated, variants in genes associated with bone marrow failure predisposition were the most frequent. Interestingly, variants in ERCC2, which encodes a protein involved with repair of damaged DNA, were recurrent among infants with KMT2A-r ALL. Future directions include comparison to germline variants in cancer predisposition genes in other infant and non-infant ALL cohorts.
Brown:Novartis: Membership on an entity's Board of Directors or advisory committees; Jazz: Membership on an entity's Board of Directors or advisory committees; Servier: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees. Guest:Syndax Pharmaceuticals: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal